LEARNING OBJECTIVES
-
- Distinguish between pure substance and mixtures:
- A pure substance has a definite set of characteristic properties (density, mp, bp), whereas a mixture exhibits properties that are a mixture of the properties of the substance they contain.
- A pure substance is composed of one kind of particle, whereas mixtures contain more than one kind of particle.
- Describe how one could use differences in characteristic properties to separate the components of a mixture.
- Sketch particle diagrams that distinguish compounds, elements and mixtures.
- Distinguish elements from compounds in terms of differences in their properties.
- Cite the evidence that supports the belief that some pure substances are “compounded” of simpler particles in a definite ratio.
- Cite evidence for Avogadro’s Hypothesis. Use this with evidence from combining volumes to deduce the formulas of some compounds.
- State features of Dalton’s model of the atom; use composition by mass data to account for the laws of definite and multiple proportions.
- Describe the evidence that supports the idea that the simple particles have a property we call charge.
- Describe the evidence that led Thomson to suggest that the mobile charge in atoms is negative.
- Use the Thomson model of the atom to account for the fact that neutral atoms can become either positively or negatively charged by the loss or gain of electrons.
- Identify properties that distinguish metals from non-metals.
- Describe the evidence that distinguishes ionic from molecular or atomic solids.
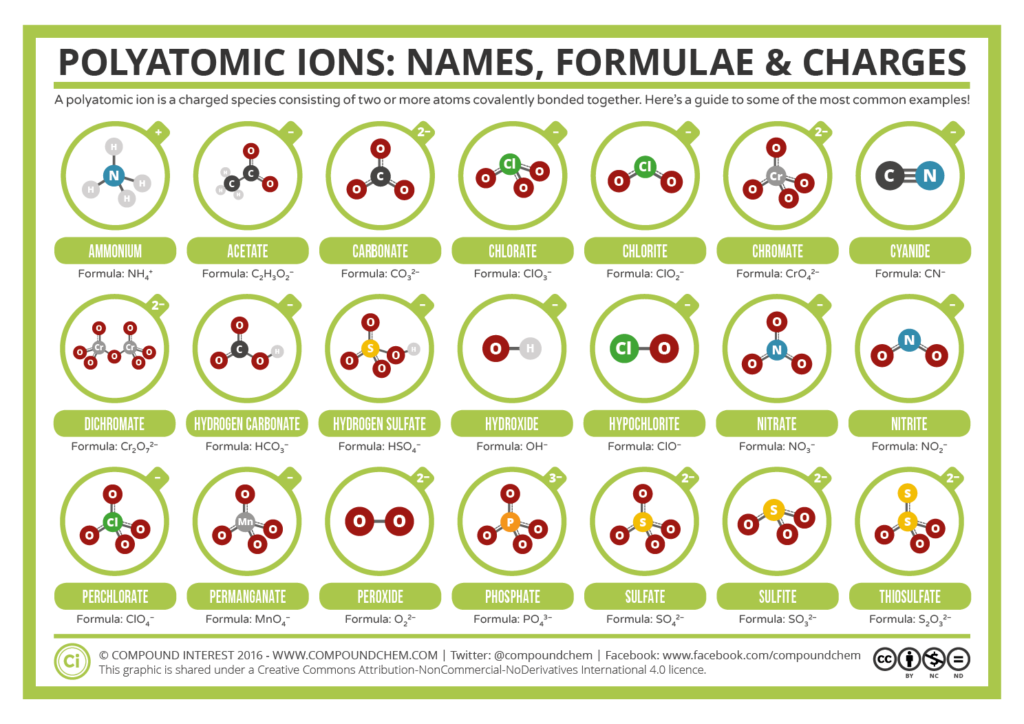
- Given the formula of an ionic or molecular substance, state its name.
- Given the name of ionic or molecular substance, write its formula.
- From the name or formula of a substance determine whether that substance is ionic or molecular.
- Distinguish between pure substance and mixtures:
NOTES
VIDEO
The Ring of Truth:
Gases & How They Combine
Option 1 Classifying Matter
Option 2 Classifying Matter
Homogeneous vs. Heterogeneous Mixtures
Distillation
Electrolysis
Mixtures & Compounds
Separating the Seemingly Inseparable
Fractional Distillation of Crude Oil
Percent Composition
The Periodic Table
Dalton’s Atomic Theory
Cathode Ray Tube
Chemical Families
Distinguishing Ionic, Molecular & Atomic Solids
Magnetic Induction
Valence Electrons & The Periodic Table
Writing Formulas for Ionic Compounds
Crossing Down Charges
Naming Ionic Compound with Polyatomics
Naming Ionic Compounds with Transition Metals (Roman Numerals)
Naming Covalent (Molecular) Compounds
En français:
Le modèle atomique de Dalton
L’organisation de la matière
Composés ioniques vs moléculaires
Nomenclature des composés ioniques
Nomenclature des composés moléculaires (covalent)
Les propriétés des composés ioniques
En español:
Elemento, Compuesto o Mezcla (Homogénea o Heterogénea)
El Modelo atómico de Dalton
SIMULATIONS
Dalton’s Playhouse
Lewis Dot Structures
PhET: Balloons & Static Electricity
PhET: Faraday’s Law of Induction (How Electricity is Generated! Super cool! Magic!)
Gizmo: Ionic Bonding
PBS Bonding
WEBSITES
Practice Classifying Matter
Element Quiz
The Mystery of Matter
JJ Thompson’s Discovery of the Electron