LEARNING OBJECTIVES
- Relate the color of the light emitted by a hot metal to its thermal energy.
- Distinguish the line spectra of light emitted by atomic gases from the continuous spectra emitted by hot metals.
- Students will be able to:
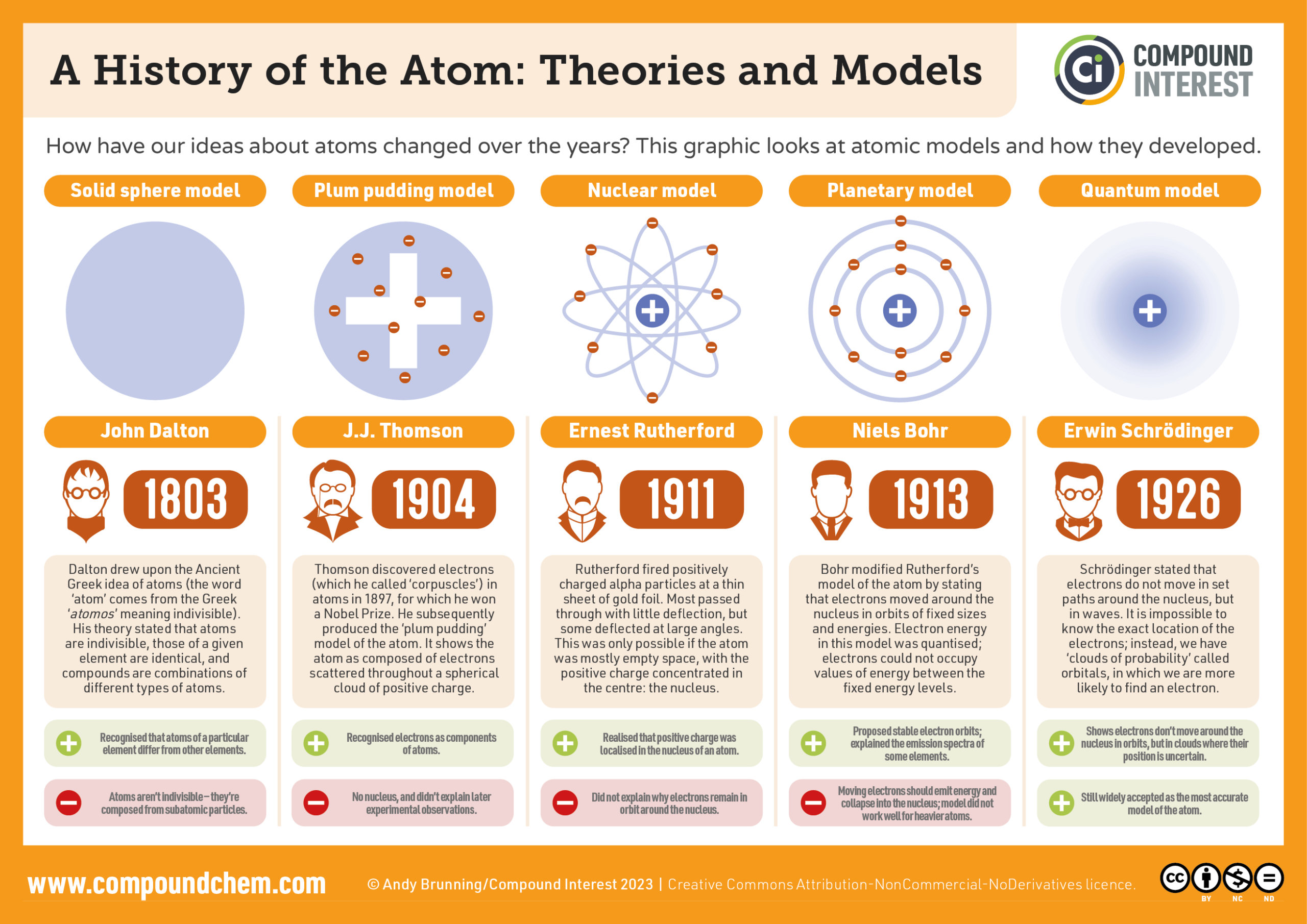
- draw the models of the atom proposed by Thomson, Rutherford and Bohr
- state the problem with each of the previous models
- describe the experimental evidence that led to a change in the previous model
- recognize which features of the new model were supported by the evidence
- recognize which features of the new model were “invented” to complete the model
- Have students will familiarize themselves with the research of Millikan, Moseley, Chadwick and Marie Curie that will enable them to:
- describe the experiments they conducted
- explain how their evidence helped to provide more detail to an existing model of the atom
- Explain how a mass spectrometer is used to determine the relative abundance of isotopes of an element. Use relative abundance and mass number to determine the average molar mass of an element.
- Relate the isotopic symbol for an element or ion with the number of protons, neutrons and electrons in that species.
NOTES
VIDEOS
Counting Subatomic Particles
Calculating Average Atomic Mass
History of Atomic Theory
Millikan’s Oil Drop Experiment
Drawing Bohr Models
Emission Spectra and Bohr Model
Electron Configurations
Electron Configuration Song
Magnetic Induction
Valence Electrons & The Periodic Table
Noble Gas Electron Configuration
Orbital Configurations
SIMULATIONS
PhET: Build an Atom
Concept Builder: Counting Subatomic Particles
Gizmo: Element Builder
PhET: Isotopes and Atomic Mass
PhET: Rutherford Scattering
Gizmo: Intro to the Bohr Model
PhET: Faraday’s Law of Induction (How Electricity is Generated! Super cool! Magic!)
Concept Builder: Electron Configurations
Gizmo: Electron Configurations
WEBSITES
SONGS
Nuclear Decay
Half Life Problems
Alpha Decay
Beta Decay
Gamma Decay
Gizmo: Radioactive Decay
Nuclear Decay Concept Builder