LEARNING OBJECTIVES
- Adapt the Bohr model for hydrogen to many-electron atoms.
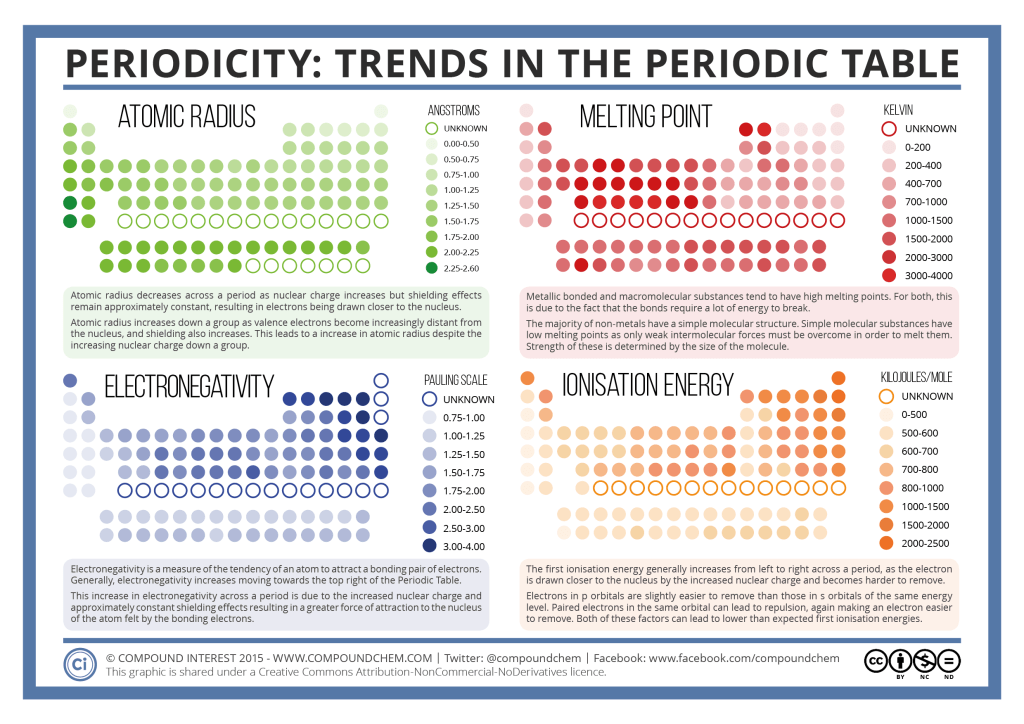
- Identify periodic trends in ionization energy and atomic size. Explain these in terms of the men-in-well model.
- Recognize that the adjective valence describes the electrons that are most readily lost during successive ionizations. Identify the number of valence electrons in atoms of the “representative elements”.
- Recognize that at normal temperatures and pressures, for non-metals, the maximum number of valence electrons per atom is eight (with the exception of two for H and He).
- Use the men-in-well model and the difference in attraction of valence electrons by the nuclei of metal and non-metal atoms to explain the formation of binary ionic compounds.
- Show that the men-in-well model is not adequate to represent the formation of a covalent bond between non-metal atoms.
- Compare strengths and weakness of various ways (molecular, line, structural) to represent covalent molecules.
- Use Lewis diagrams to represent the sharing of valence electrons to form covalent bonds in compounds.
NOTES
VOCABULARY
VIDEO
Locating Electrons by Successive Ionization Energy Periodic Trends Men in Wells Model Ion Formation How to Draw Lewis Structures Alkali Metals React with Water Alkali Metals Alkaline Earth Metals Transition Metals Gallium Rare Earth Metals Halogens Noble Gases Most Dangerous Elements Periodic Trends Ionic Radius Trends Periodic Videos Animated Element Song Crash Course: Polar vs. NonPolar VSEPR Theory Intro VSEPR Theory Practice Problems VSEPR Theory Common MistakesSIMULATIONS
PhET: Molecule PolarityWEBSITES
Website: Periodic Videos (for each element) The Many Looks of the Periodic Table Discovery of the 1st ElementINFOGRAPHICS
Group 1 – Alkali Metals Group 2 – Alkaline Earth Metals Transition Metals Group 3 – Icosagens Group 4 – Crystallogens Group 5 – Pnictogens Group 6 – Chalcogens Group 7 – Halogens Group 8 – Noble Gases Lanthanides ActinidesSONGS