LEARNING OBJECTIVES
- Describe the evidence that supports the idea that the simple particles have a property we call charge.
- Describe the evidence that led Thomson to suggest that the mobile charge in atoms is negative.
- Use the Thomson model of the atom to account for the fact that neutral atoms can become either positively or negatively charged by the loss or gain of electrons.
- Identify properties that distinguish metals from non-metals.
- Describe the evidence that distinguishes ionic from molecular or atomic solids.
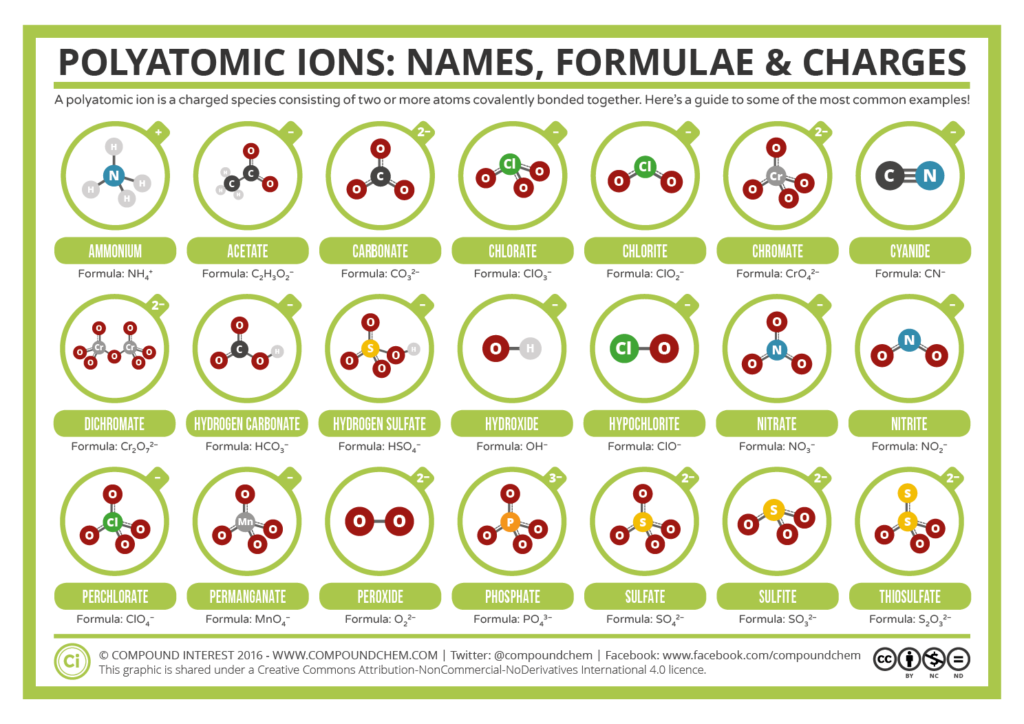
- Given the formula of an ionic or molecular substance, state its name.
- Given the name of ionic or molecular substance, write its formula.
- From the name or formula of a substance determine whether that substance is ionic or molecular.
NOTES
VIDEOS
Cathode Ray Tube
Chemical Families
Distinguishing Ionic, Molecular & Atomic Solids
Magnetic Induction
Valence Electrons & The Periodic Table
Writing Formulas for Ionic Compounds
Crossing Down Charges
Naming Ionic Compound with Polyatomics
Naming Ionic Compounds with Transition Metals (Roman Numerals)
Naming Covalent (Molecular) Compounds
En français:
Composés ioniques vs moléculaires
Nomenclature des composés ioniques
Nomenclature des composés moléculaires (covalent)
Les propriétés des composés ioniques
SIMULATIONS
PhET: Balloons & Static Electricity
PhET: Faraday’s Law of Induction (How Electricity is Generated! Super cool! Magic!)
Gizmo: Ionic Bonding
WEBSITES
JJ Thompson’s Discovery of the Electron