LEARNING OBJECTIVES
- Describe properties of aqueous solutions of acids and bases.
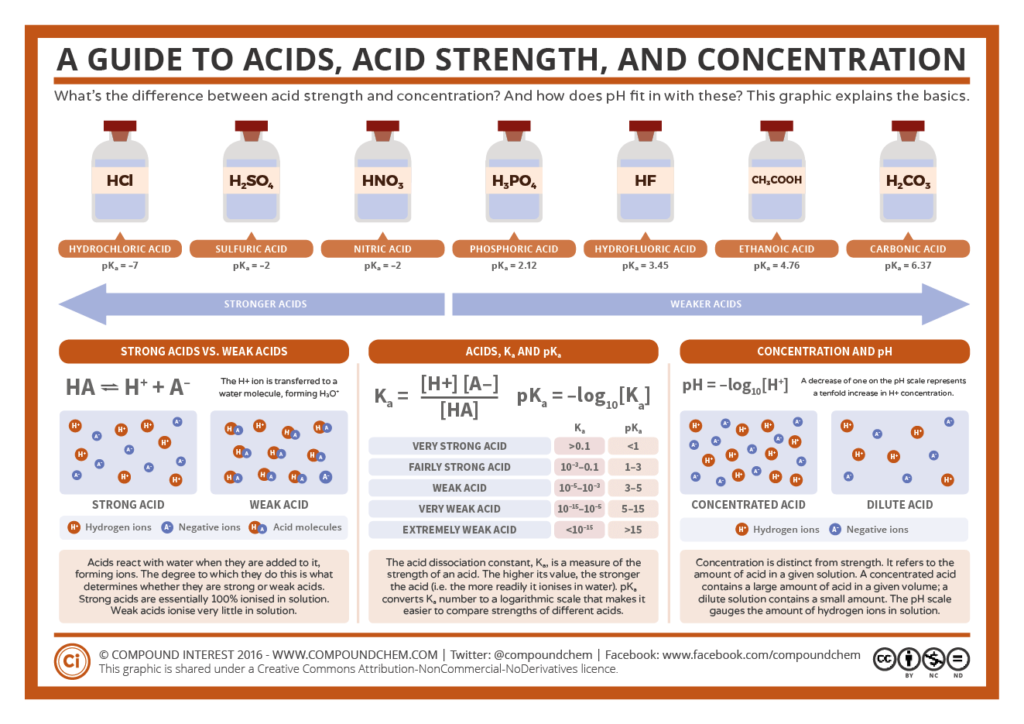
- Account for differences between acids and bases in terms of the Arrhenius model.
- Use the Bronsted-Lowry model of acids and bases to identify the proton donor, proton acceptor, conjugate acid and conjugate base in a given equation.
- Describe strength of weak acids and bases in terms of the extent to which they compete with water for H+ ions.
- Distinguish “concentrated” from “strong” and “dilute” from “weak” as these terms are used to describe acids and bases.
- Given the mass (or number of moles) of a known strong acid or strong base and the total volume of solution, calculate the [H3O+] and [OH–].
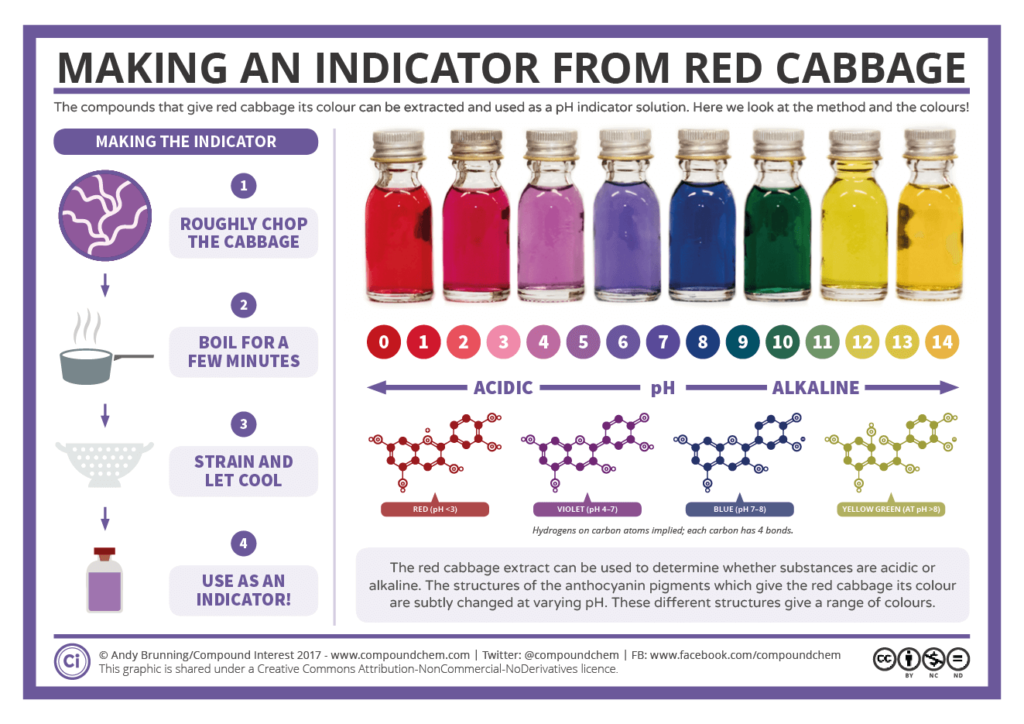
- Describe indicators as weak acid/base mixtures whose acidic and basic forms have different colors.
- Given the volume and concentration of known acid (or base) used to titrate a base (or acid), calculate the concentration of the unknown solution.
- Recognize that pH is a way of describing the [H3O+] of solutions using a logarithmic scale. Given the [H3O +] or pH, calculate the other.
- Identify the endpoint of a titration as the point at which the rate of change of [H3O+] is greatest.
NOTES
VIDEOS
Naming Acids & Bases
Acids, Bases & The pH Scale
pH/pOH Calculations
Neutralization Reactions
Acid-Base Reactions in Solution
Titration Problems
SIMULATIONS
PhET: Acid/Base Solutions
PhET: pH/Dilution/Concentration
Gizmo: pH Analysis
Gizmo: pH Analysis: Quad Color Indicator
Gizmo: Titration
RCS: Titration Screen Experiment
WEBSITES
Acid Base Properties Concept Builder
Dissociation Concept Builder